What is phenolphthalein. The main hazards in steam distillation.
(Document)
n2.doc
Federal Agency for Education
General experiment 1: titration
Why should you make sure your eyes are even with a mark when you add the last few drops of water?
- What is the concentration of this standard solution?
- What is the purpose of washing the cup and funnel with water?
- What is the purpose of flushing the inside of the volumetric flask with water?
Hot Alcohol Recrystallization Technique
From the table, determine the average titration volume for this experiment and use this value in your other calculations. Using the previous examples, determine the concentration of acetic acid in a vinegar sample. Remember that you have the following information: the volume of the vine volume of the sodium hydroxide solution, the concentration of the sodium hydroxide solution. Determine the concentration of sodium hydroxide solution with unknown concentration.
State educational institution of higher professional education
Pomor State University named after M.V. Lomonosov
Department of Chemistry
Course work
Synthesis of phenolphthalein.
Solubility study in solvents with different dielectric constant
Made by:
Concentrated acids and bases can cause severe burns. We suggest using gloves and goggles when working with acid or base. After making sure that the burette is closed, carefully add the standard oxalic acid solution to the burette until the burette is full. Place a clean glass under the burette and carefully place part of the oxalic acid solution in it. This will ensure that there are no air bubbles in the burette. The upper part of the solution should now be between the marks 0 and 1 cm 3. Write down the value to the second decimal place. Use a pipette to measure a 20 cm 3 solution in a conical flask. Add 3-4 drops of phenolphthalein indicator to the conical flask. Carry out a crude titration experiment by quickly adding oxalic acid to the conical flask and constantly twisting the conical flask. Stop as soon as the color of the solution changes, and after swirling it will change. If the color change does not remain when the flask is twisted, add more oxalic acid until the color remains. Repeat steps 4-7 with the second conical flask. Quickly add oxalic acid to the conical flask until you add 2 cm 3 less than your coarse titration volume. At the moment, there should be no lasting color change. Add oxalic acid gradually. Swirl between each drop and, if necessary, rinse the sides of the flask with water. When the solution changes color and remains a new color, pay attention to the volume on the burette. Remember that titrated volume: -. For accuracy, repeat steps 9-11 until you have three readings with a difference of no more than 0, 1 cm 3.
- Mark one of the glasses \\\\.
- Use a pipette to measure 100 cm 3 of water in a glass.
- Add approximately 4 g \\\\ to the glass and mix.
- Attach the burette to the retort stand and place a small funnel on top.
- What color was the sodium hydroxide solution when phenolphthalein was added?
- What was the color when enough acid was added?
3 year student
chemistry departments
Alferov Vladimir Ivanovich
Checked:
Associate Professor Chem. of sciences
Lewandowska T.V.
Associate Professor tech. of sciences
Chagina N. B.
ARKHANGELSK
1. Literature review ………………………………………………… ..5
Appearance and physical properties of phenolphthalein ………… .5
The use of phenolphthalein …………………………………… ... 6
Chemical properties ………………………………………………… ... 8
Getting phenolphthalein ……………………………………… 11
1.4.2 Physical properties of substances used in the synthesis of ... .15
Using the previous examples, determine the concentration of sodium hydroxide solution. Remember that you have the following information: the volume of the sodium hydroxide solution in the volume of the oxalic acid solution is the concentration of the oxalic acid solution. A balanced chemical equation for this reaction.
Calculate the mass of oxalic acid that the student must dissolve in order to make the required standard solution. He believes that 40 cm 3 of oxalic acid solution completely neutralizes 35 cm 3 of sodium hydroxide solution.
- We need a lot of oxalic acid.
- However, we do not yet know the number of moles.
1.4.3 Methods of steam distillation ……………………… .16
1.4.4 Procedure for recrystallization from hot alcohol .............. 18
1.5 Safety precautions ………………………………………………… 20
1.5.1 Main hazards when working with concentrated acids ...................................................................................... 20
1.5.2 Main hazards during steam distillation ........ 21
- Write a balanced chemical equation for the reaction.
- Calculate the acid concentration.
This rain, often acidic, was first noted in the nineteenth century, but only until the twentieth century did this acidity become widespread at dangerous levels, which we associate with the term “acid rain”. The main culprit was coal containing sulfur as an impurity. When burned in a power plant, this sulfur combines with oxygen to form sulfur dioxide, which rises through the chimney and dissipates through the atmosphere.
1.5.3 Main hazards during recrystallization from hot alcohol ...................................................................
1.5.4 Features of work with substances used in the synthesis of …………………………………………………………………………… 22
1.6 Characterization of solvents ………………………………… .24
1.6.1 Classification of solvents by physical properties ... 25
Sulfuric acid is a very strong acid, and when water condenses from the air to form raindrops, the acid dissolves in water. Then the rain falls and collects in the bodies of fresh water, increasing their acidity. The northeastern United States has been hit hardest by this because the Ohio Valley, including the Ohio and Pennsylvania districts, are places with most of the coal industry of the past century and a half. Due to prevailing weather conditions, these flue gases enter the atmosphere in the Ohio Valley, but rain falls in the north of New York State and New England.
1.6.2 Solvent classification systems based on their chemical properties ………………………………………………… ..30
1.7 Overview of methods for determining the solubility of solids .......................................... 35
1.7.1 Gravimetric method ……………………………………… .35
1.7.2 Electrochemical method …………………………………… 37
1.7.3 Photoelectrocalorimetric method …………………… ..41
An increase in rain acidity can be devastating. Metal impurities in the soil, which are usually tightly bound to other compounds, can be washed with acid rain in lakes and streams; at one time, many ponds in New England were unable to maintain trout trout because of this. Plants are also very sensitive to soil pH. beneficial nutrients can also be washed out of the soil, leaving plants unable to survive in it. In particularly severely affected areas, dozens of dead trees can be found, as shown above.
2. The experimental part …………………………………………… 43
2.1 Synthesis of phenolphthalein ………………………………………… ... 43
2.2 Recrystallization from hot alcohol ……………………… ..45
2.3 Determination of solubility …………………………………… .47
Conclusion ................................................ 50
Bibliographic list ………………………………………… .51
Buildings and statues were also damaged by acid rains that eat up stone, especially limestone. Increased erosion of sculptures, tombstones and even some buildings led to huge losses - some works of art were completely destroyed.
Since then, emissions have been significantly reduced, and therefore acid rain has also decreased. However, the problem has not yet been resolved, and much remains to be done on this serious environmental problem. Let's look at hydrochloric acid, which is the main component of the acid in the stomach, and sodium hydroxide, which is often referred to as the “alkali,” and is used in soap. However, almost all hydronion and hydroxide combine to make water molecules, leaving behind only the chloride-spectator and sodium ions.
Introduction
Phenolphthalein is one of the most widely used acid-base indicators in chemistry. It belongs to triarylmethane dyes called phthaleic.
The aim of this work is the synthesis of phenolphthalein and the study of its solubility in solvents with different dielectric constants. To achieve this goal, it is necessary to solve the following tasks:
If the amount of hydrochloric acid is exactly the same as the amount of sodium hydroxide in moles, then each hydronium must have a hydroxide in order to react with leaving a solution that is currently neutral. it should now be a glass of clean water with a pH of 7 and ordinary table salt dissolved in it. We start with two extremely caustic substances and end with benign products.
In the general case, when acids and bases interact with each other, they neutralize each other and the pH of the solution approaches this. It is called ".". Only when an equal amount of strong acid and strong base are added together, the pH will be accurate. But in many cases, weak acids and weak bases combine. However, the pH will approach 7, but the solution will not be neutral. In the case of acid rain, stones and soil are often the main, especially carbonate rocks found in parts of the country.
1. Analyze the literature on this issue;
2. Choose synthesis methods and choose the most acceptable of them;
3. To carry out the synthesis, isolation and purification of the product;
4. Investigate the solubility in solvents with different dielectric permittivities, compare the results with literature data;
Since the weak acids in acid rain fall on the rock, they are neutralized by bases on the leaves, in the mud and in the rocks. However, many of these bases, for example, in the rocks, are not renewable. After a century of acid rain, rocks neutralize the entire base in the rocks, acid rain simply enters the ecosystem, and nothing neutralizes it.
Phenolphthalein: a common laboratory indicator
The phenolphthalein molecule is transparent in the presence of acid and pink in the presence of a base. This is an excellent indicator for finding the equivalence point in acid-base titration for simple acids. In Section 5 of Section 8, titrations were introduced as a way of combining the two solutions together to have a controlled stoichiometric reaction. It requires a solution of known concentration, usually in a burette, which will react with a solution of unknown concentration in a glass underneath. Any reaction will work for titration, but in order to be able to tell when the reaction will be completed, there must be a visible endpoint.
5.
Make a conclusion about the work done.
1. Literature review
1.1 Appearance and physical properties of phenolphthalein
Phenolphthalein (Di-n-dioxidiphenylphthalide, 2,2-bis (n-hydroxyphenyl) phthalide) is a white or slightly yellow crystalline powder; upon recrystallization from diluted ethyl alcohol, colorless, rhombic needles are formed. Phenolphthalein is odorless, not stable in air. The molecular weight is 318.3 g / mol. Phenolphthalein melts at a temperature of 250 ° C (258 ° C) and sublimes at higher temperatures.
Phenolphthalein as a means for losing weight
For reactions with an acid base, indicators can provide precisely this type of color-changing information to indicate the end point. Therefore, acid base neutralization reactions are one of the most common types of reactions used in titration assays. Thus, titration can be used to determine the amount of acid or base in an unknown solution.
When titrating strong acids with strong bases, the end point is reached when the pH is exactly 7, because the solution should be just a pure aqueous solution with neutral electrolytes in it. After the acid disappears, any base that we add to the flask will not be neutralized, and the pH of the solution will begin to increase. However, even a small fraction of a drop from an excess strong base burette will lead to a change in pH. One or such indicator, phenolphthalein, is transparent and colorless in acidic solutions, but in basic solutions it becomes dark pink if the pH is above 2.
Phenolphthalein is soluble in ethanol, diethyl ether, chloroform, acetone, petroleum ether, benzene, toluene and poorly in water.
1.2 Use of phenolphthalein
Phenolphthalein is one of the most widely used indicators, especially in the volumetric determination of weak acids.
It has several advantages: it is not sensitive to elevated temperatures, the error from the presence of protein substances and colloids is insignificant. It can be widely used even in solutions containing alcohol, only the color of the alkaline solution differs from the violet tone characteristic of an aqueous solution. In concentrated alcohol solutions, the main form has a blue-violet color. Phenolphthalein can be used in the titration of organic acids in alcohol solutions or for determining the acidity of alcohols and esters. Hayort and Ham propose the use of disodium glycolate to establish a titer of bases in the presence of phenolphthalein. Satisfactory results are obtained by titration of 2 · 10 -6 - 30 · 10 -6 g of organic acids in a volume of 2.7 · 10 -6 - 40 · 10 -6 l in the presence of phenolphthalein. According to Mick's studies, the concentration of the indicator plays a very important role in microacidimetry. Phenolphthalein is part of many mixed indicators. Due to its qualities, it is suitable for calorimetric determination of pH using buffer solutions or according to the Michaelis method without buffer solutions. The colored form obeys Beer's law in a wide range of concentrations.
Acid indicators come in many types
The fact that Phenolphthalein changes color slightly above 0 usually leads to a very small error in the experiment. In addition to phenolphthalein, other indicators include bromotimol blue and methyl orange. For solutions that differ only slightly from their endpoint, bromothymol blue is a good indicator. Just below pH 7, the solution has a yellow color that turns blue just above pH. When the titration endpoint is quite acidic, methyl orange is used.
Phenolphthalein can also be used as a reagent in a qualitative analysis. According to Sachs, some insoluble metal hydroxides, such as lead, cadmium, zinc, magnesium, etc., when sprayed with phenolphthalein give a color, which is explained by the adsorption of the indicator. Another group of reactions is based on the fact that in an alkaline medium phenolphthalein is reduced by zinc to colorless phenolphthalein. This leuko compound is oxidized by some substances, and then red phenolphthalein appears again. A similar effect is observed with very small amounts of cyanide ions (0.01-0.05 mg / l) in the presence of traces of copper sulfate. At very small amounts of copper (10 -4 - 10 -5%), the introduction of hydrogen peroxide into the system leads to an increase in the rate of oxidation of the leuko compound.
One of the phenolphthalein derivatives - phenolphthalein phosphate - is used in determining the activity of the phosphatase enzyme. Phenolphthalein phosphate alone does not have the properties of an indicator, but is destroyed by the action of the enzyme; phosphate groups are cleaved off by the action of the substrate, and a red color of phenolphthalein appears.
In medicine, phenolphthalein is used as a laxative, and the drug is called - purgen.
1.3 Chemical properties
The phenolphthalein coloration transition range is from pH 8.2 (colorless) to pH 9.8 (purple). To determine the transition interval, a series of buffer solutions with the following pH values \u200b\u200bis used: 7.8-8.0-8.2-8.4-9.0-9.6-9.8-10.0-10.2. The pH values \u200b\u200bat which the first pink color appears are dependent on the concentration of the indicator. The intensity of the purple color gradually increases up to pH 9.8: pH 1/2 9.53.
The process of formation of a colored dianion of quinoid structure (III), which is formed as a result of the interaction of phenolphthalein with a dilute alkali solution (pH 8.5), can be represented as follows:
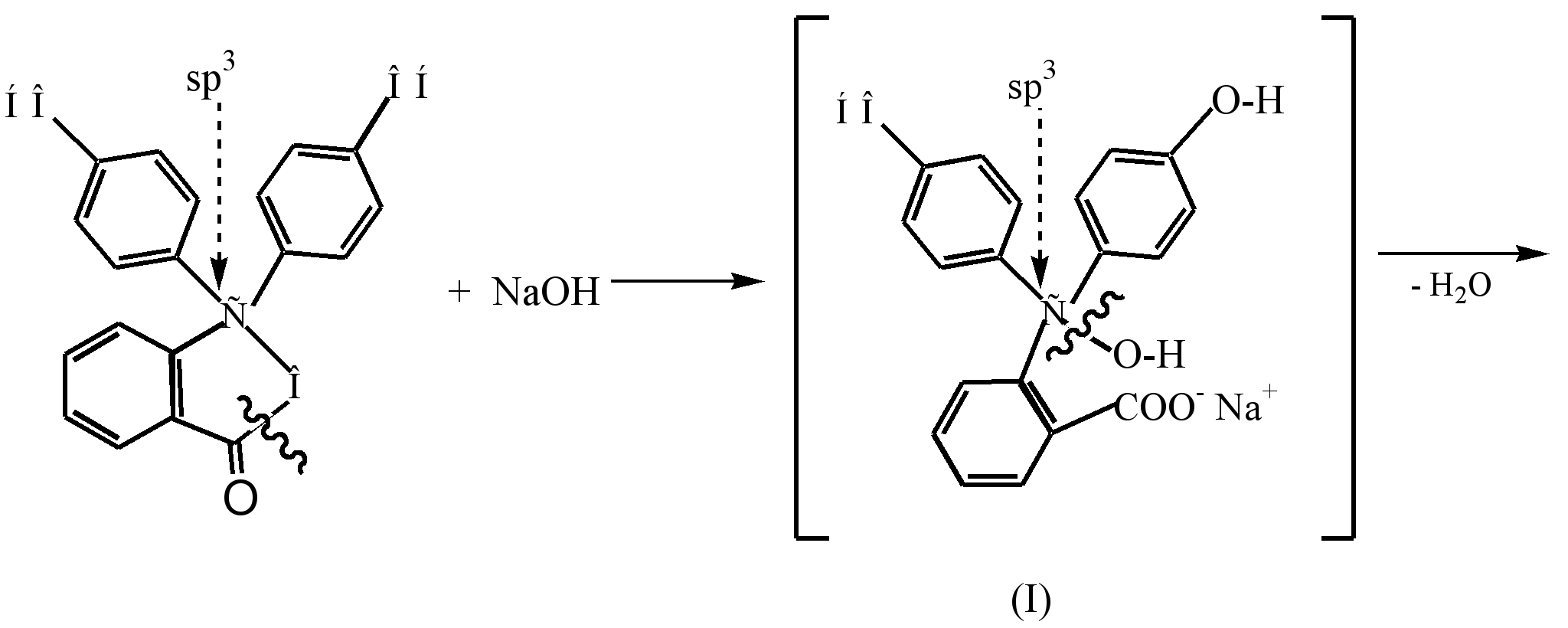

Under the action of alkali in the colorless phenolphthalein, the β-lactone ring is opened (hydrolysis), and the resulting colorless alcohol (I) spontaneously cleaves a water molecule to form a yellow monosodium salt (II). Further interaction with alkali leads to the formation of disodium salt (III) and to a deepening of the color to raspberry, which is due to the lengthening of the conjugation chain.
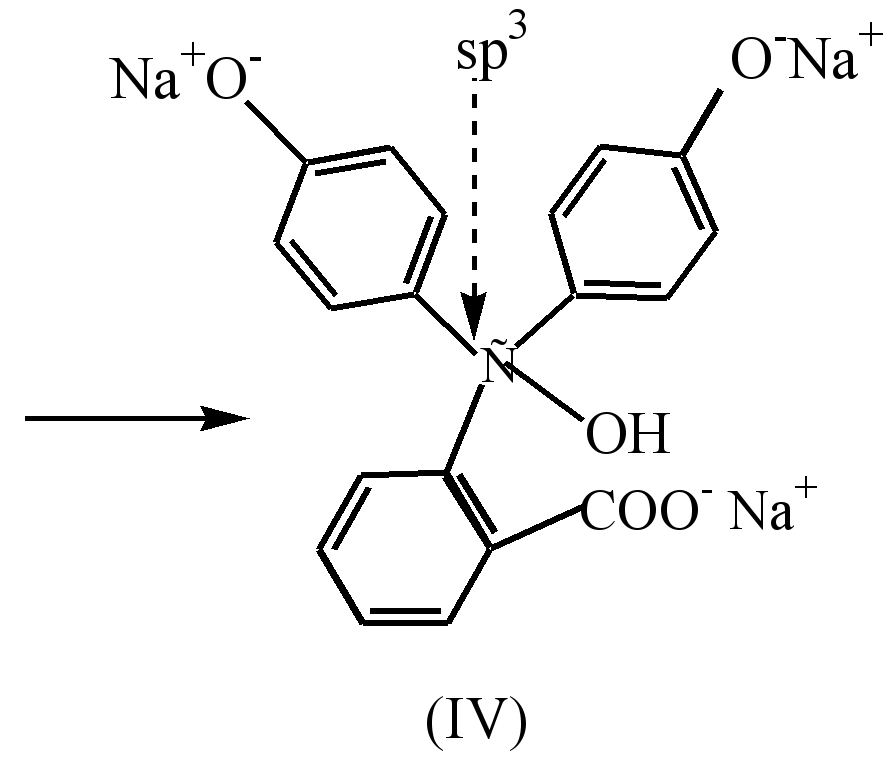
In a strongly alkaline medium (pH-10), discoloration of the solution is observed as a result of the transition of the disodium salt (III) to the trisodium salt (IV), in which the rings are not conjugated.
In an acidic environment, it is colorless, since weak benzene chromophores are isolated, autonomous, and - interaction (pairing) with each other does not enter:
Are they disconnected With sp 3 - a carbon atom that is an insulator and not a conductor? - electrons. In an alkaline medium, the lactone cycle breaks, one of the phenolic hydroxyls ionizes, and the C sp 3 atom takes on the C sp 2 configuration, becoming a conductor? - electrons between benzene chromophores, moreover, polarized auxochromes - СОО - and –О -. In concentrated sulfuric acid, the indicator turns orange. The color intensity of purple alkaline solutions when standing decreases partly due to the formation of a colorless carboxy base (this is a reversible transformation), partly due to irreversible oxidation by air
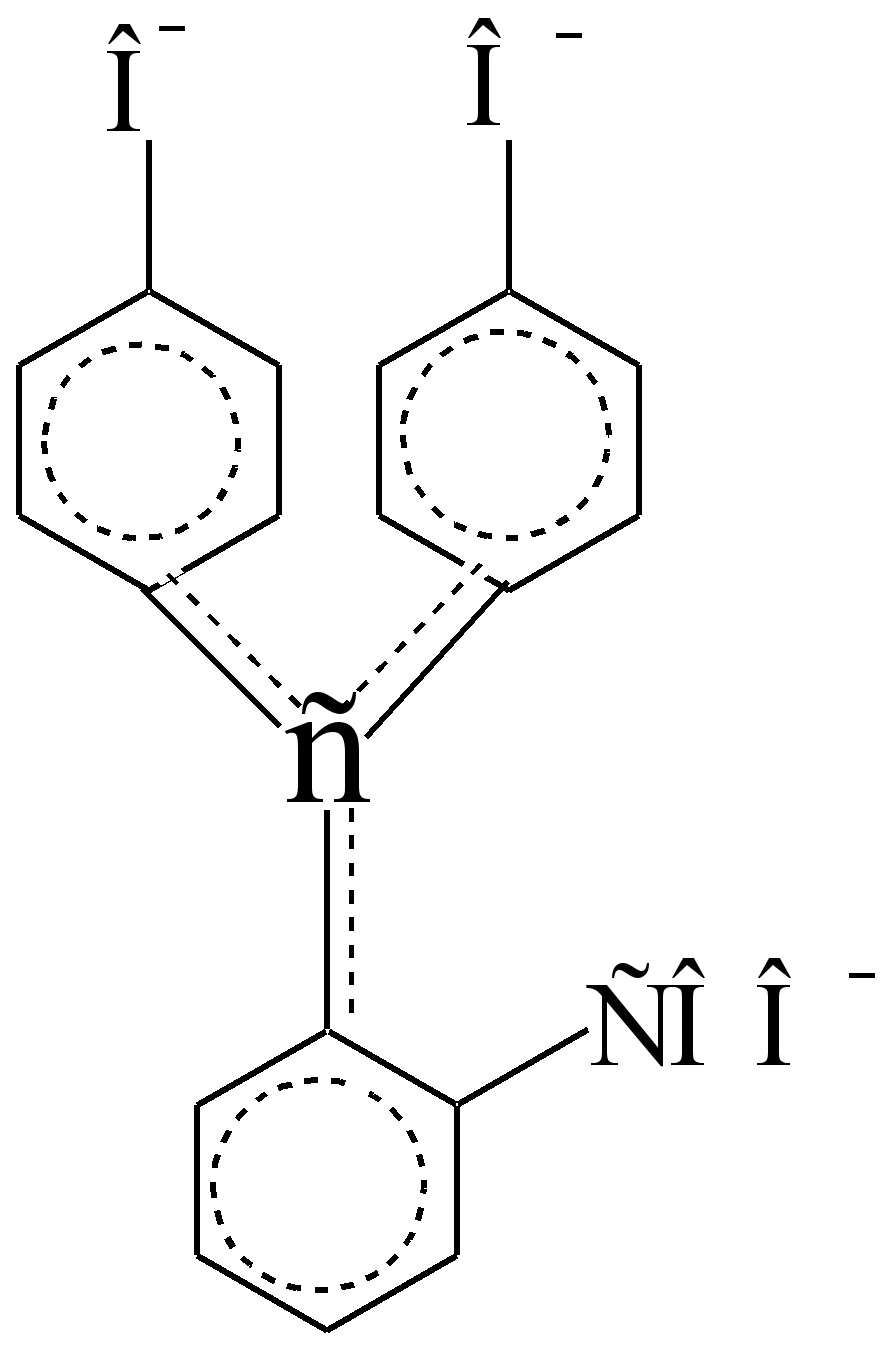
Phenolphthalein Chromophore
(alkaline form, 1 \u003d 553 nm, 1 \u003d 9000)
1.4 Obtaining phenolphthalein
The main method of producing phenolphthalein is the condensation of phenol with phthalic anhydride. This reaction is a special case of Friedel-Crafts acylation. The reaction proceeds when heated with an acid catalyst (concentrated sulfuric acid or zinc chloride):
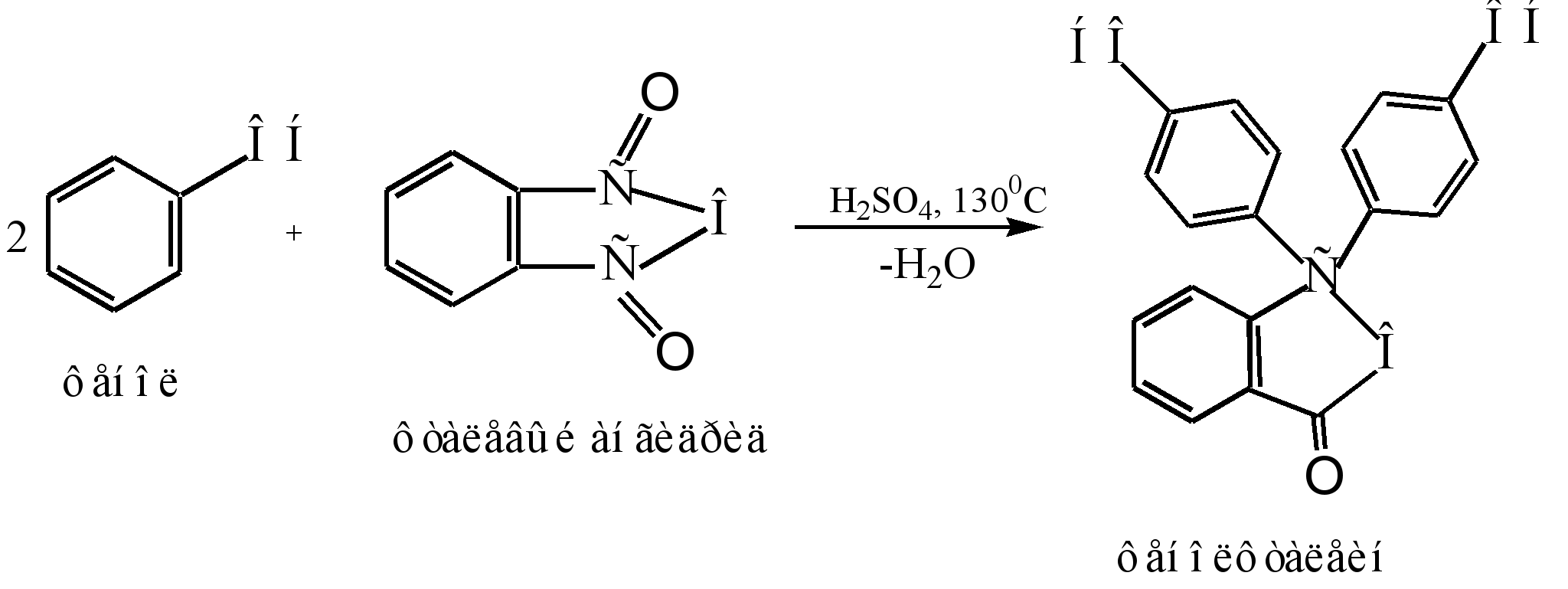
The reaction mechanism can be represented as follows:

Sigma - complex
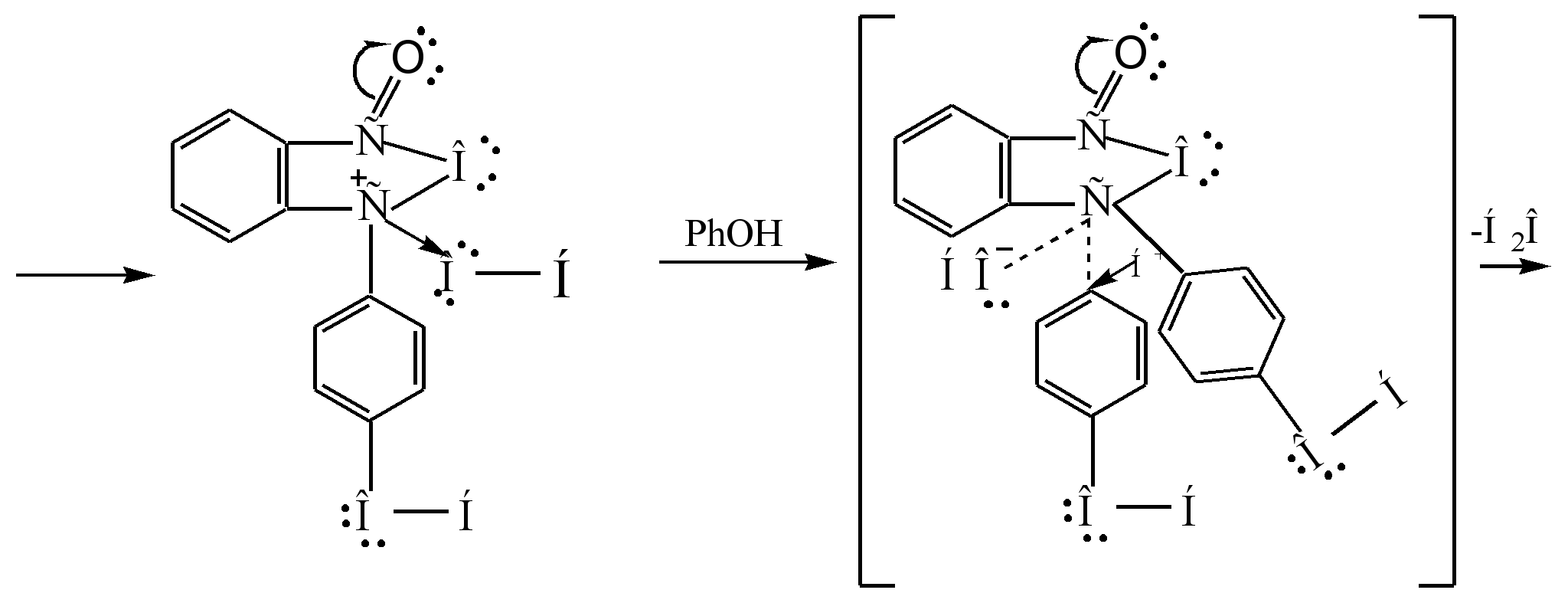
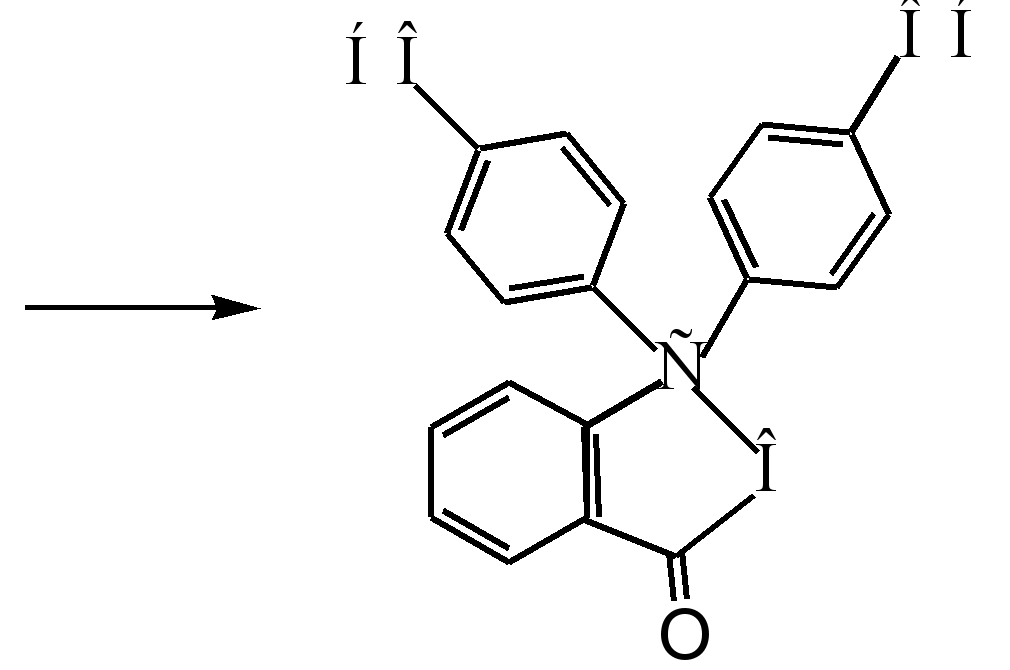
phenolphthalein
1.4.1 Methods for the production of phenolphthalein
After analyzing the literature on this issue, it was found that in all methodological manuals the same methodology for obtaining phenolphthalein is given. It is based on the reaction presented above. The technique is fully suited to our laboratory conditions.
For the synthesis you will need the following reagents, dishes, and equipment:
Phthalic anhydride-5g; phenol-10g; sulfuric acid - 2 ml; sodium hydroxide - 4-5%; acetic acid (concentrated); hydrochloric acid (concentrated); ethyl alcohol (absolute).
Round-bottom flask (50ml) - 1 pc; glass (100ml) - 1 pc.; porcelain cup - 1 pc.; conical flasks (250ml) - 2 pcs.; thermometer; steam distillation device; sand bath.
Stroke synthesis:
A mixture of 5 g of phthalic anhydride, 10 g of phenol and 2 ml of sulfuric acid is placed in a flask and heated in a sand bath for 1.5 hours, strictly observing a temperature of 125 - 130 ° C (a thermometer in the melt and periodically mixing the contents). The hot mixture is poured into a flask with 100 ml of boiling water, distilled off with steam, unreacted phenol. To avoid splashing and the associated loss of substance, the liquid must be mixed all the time. The end of the distillation can be seen by the change in the turbidity of the distillate: if the distillation flows unclear, then distillation should be continued, if the distillation is transparent, then distillation can be stopped. The solution was allowed to cool, after which it was decanted onto a Buchner funnel, being careful not to transfer the precipitate to the filter. Then the product is washed by decantation with two small portions of cold water, attach what still fell on the filter to the bulk of the solid in a glass, dissolve in a small amount of warm 4-5% sodium hydroxide and unreacted phthalic anhydride is separated by filtration. Acetic acid is added to the dark red filtrate until the phenolphthalein is completely precipitated, then 1-2 drops of hydrochloric acid are added and left for 1 hour.
The released phenolphthalein in the form of yellowish sand is sucked off on a Buchner funnel. The crude product is transferred to a beaker and dissolved by heating in approximately 10 ml of alcohol. The hot solution is sucked off and the filter cake washed with hot alcohol. Filter through an alcohol-moistened filter to remove droplets of oil. The filtrate is transferred to a porcelain cup and heated in a hot sand bath to remove a significant portion of the alcohol. The mixture in the cup is left to stand for 30 minutes. The isolated colorless or light yellow crystals are sucked off and dried in air between sheets of filter paper.
1.4.2 Physical properties of substances used in the synthesis
Table 1
Physical properties of substances used in the synthesis
| Title | Formula | appearance | T pl, 0 C | T bale, 0 C | solubility |
|
| In water | in a different |
|||||
| Phenolphthalein | C 20 H 14 O 4 | Colorless or sand-colored rhombic needles, odorless, not resistant to air | 261 | sublime huddles | Sparingly soluble (0.0002 g in 100 g of water) | Good ethanol, moderately in diethyl ether, also soluble in alkaline solutions. |
| Phenol | C 6 H 5 OH | Colorless or pinkish rhombic needles | 41-43 | 182 | In cold - moderately In the hot - good | Good in ethanol, ether, chloroform, glycerin, carbon disulfide |
| Phthalic anhydride | C 8 H 4 O 3 | White rhombic needles | 130,8 | Vozg 284.3 | badly | In ethanol, ether, chloroform |
| Sulfuric acid | H 2 SO 4 | Colorless, viscous liquid with an unpleasant odor | 10,37 | 330 | | reacts |
| Sodium hydroxide | NaOH | White blurry, rhombic crystals | 320 | 1378 | Soluble with heat | In alcohol, glycerin, phenol, ether, acetone |
| acetic acid | CH 3 COOH | Colorless liquid with a pungent unpleasant odor | 16,6 | 118,1 | | In ethanol, ether |
| Hydrochloric acid | HCI | Yellowish liquid, smokes in the air, with an unpleasant odor | -15,35 | - | well | In alcohol |
1.4.3 Method of steam distillation
Since, during the course of the synthesis, it will be necessary to distill off unreacted phenol using steam distillation, it is necessary to consider the procedure for its implementation.
Distillation is carried out in a device consisting of a steam generator with a safety tube, a Kleisen distillation flask (250 ml), a thermometer, a direct Liebig refrigerator, an allonge, and a receiver. A steam generator and a distillation flask connected through a tee to one of the processes of which a rubber tube with a Hoffman clip is put on, is heated in a sand bath.
The course of distillation (distillation): a hot melt is placed in a distillation flask with 100 ml of boiling water. By heating a steam generator and a distillation flask, a distillate containing unreacted phenol is collected. The turbidity of the distillate indicates its presence. The distillation is stopped at the end of the runoff of the turbid distillation, that is, until a transparent distillation appears. 
Fig. 1 Steam distillation apparatus
1- safety tube; 2 - steam supply pipe; 3 - tee; 4 - clamp; 5 - steam generator; 6 - Kleisen flask; 7 - thermometer; 8 - Liebig refrigerator; 9 - allonge; 10 - receiver; 11 - mantle heater; 12 - tile.
1.4.4 Procedure for recrystallization from hot alcohol
Recrystallization is experimentally carried out as follows: The substance is placed in a round bottom flask and a little solvent is added to it. The mixture is heated under reflux in a hot water bath heated on a tile.
Most liquids are prone to overheating, and therefore they boil with strong jolts. To avoid this, boilers are introduced into the flask prior to heating.The solvent is gradually added in small portions through a reflux condenser until the substance is completely dissolved.
After complete dissolution of the substance, the heating is stopped and the mixture is allowed to cool together with water in the bath. The precipitated substance is filtered off.
In most cases, two-stage recrystallization is a reliable way to purify a substance. 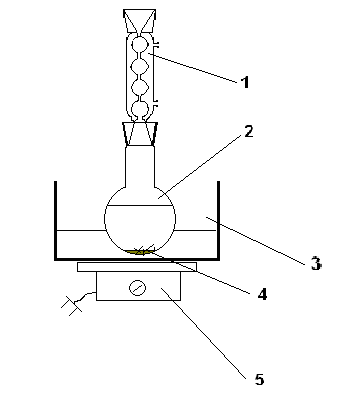
Fig.2 Recrystallization Instrument
1- reflux condenser; 2 - round bottom flask; 3 - water bath; 4 - boilers; 5 - tile.
1.5 Synthesis Safety
1.5.1 General hazards when working with concentrated acids
1. Strong inorganic acids in contact with skin cause chemical burns.For chemical burns caused by contact with concentrated acids on the skin, the burned area is washed with a strong stream of water, and then with a 1% solution of sodium bicarbonate (located in the medicine cabinet). If acid gets into the eyes, immediately rinse with 1% sodium hydrogen carbonate solution and distilled water at room temperature. Seek help from a doctor.
2. In view of the fact that concentrated hydrochloric acid soars with hydrogen chloride, it is necessary to work with it under a well-functioning draft and a slightly lower draft curtain. In case of poisoning with hydrogen chloride vapors, it is necessary to immediately go out into the fresh air.
3. Spilled acid must be dipped in sand and neutralized with solid sodium carbonate until gas evolution ceases. After complete neutralization, rinse the sand with a large volume of tap water, check for completeness of washing (make a test on Cl - using AgNO 3) and, after drying in an oven, return to the sand box.
1.5.2 Main hazards during steam distillation
The main hazards during distillation are associated with the use of electric heating devices (electric stoves, mantles).1. Cord (tiles with a cord cannot be used in rag windings), the plug and the hob must not have visible damage.
2. Do not use your hands to check the temperature of the heat-emitting surface.
3. Turn on and off the electric stove from the network by inserting and removing the plug, holding with one hand the socket, the other with the plug. Use the switch to adjust the heating.
4. In the case of a thermal burn, it is necessary to make long lotions of the burned place with a 0.5% solution of potassium permanganate, then close it with a sterile dressing and consult a doctor. In case of a burn of 3 and 4 degrees, immediately apply a sterile dressing and consult a doctor.
1.5.3 Main hazards during recrystallization from hot alcohol
1. The total volume of poured alcohol should not exceed 200 ml and should occupy no more than 1/3 of the volume of the flask.2. The size of the water bath should be larger than the heat-radiating surface of the tile.
1.5.4 Features of work with substances used in the synthesis
Most chemicals are more or less toxic. Precautions when working with them are aimed at preventing cases of penetration of these substances into the body through the mouth, lungs and skin.During the synthesis, potentially hazardous substances will be used: phenol, sulfuric acid, acetic acid and hydrochloric acid.
Phenol is a colorless (pinkish), crystalline substance with a characteristic odor. MAC in the air of the working zone 0.3 mg / m 3. The hazard class is second. All work with phenol is carried out in a fume hood. Toxic effect: nerve poison, has a strong local irritating and cauterizing effect, causing general severe poisoning. Possible death. The situation is complicated by the fact that the substance causes local anesthesia. First aid: if it comes into contact with clothing, change it immediately, if it comes into contact with skin, wash it off with vegetable oil or a solution with a mass fraction of ethanol of 10 - 40%.
Hydrochloric acid belongs to the second hazard class (MPC in the air of the working area 5 mg / m 3 in terms of HCl), all work with concentrated hydrochloric acid must also be carried out in a fume hood because it emits hydrogen chloride vapor. In case of poisoning with hydrogen chloride vapors, it is necessary to go into fresh air, other hazards are indicated above.
Sulfuric acid ( \u003d 1.84 g / cm 3) - MPC pz \u003d 1 mg / m 3. The hazard class is second. After about 5 seconds, energetic dehydration of the tissues begins, where the drop fell. First aid: rinse the skin with running water, followed by neutralization.
Acetic acid - MPC rz \u003d 5 mg / m 3. Hazard Class Three 7. Vapors irritate the upper respiratory tract, solutions with a mass fraction of more than 30% act on the skin, and on the eyes more than 2%. First aid: (see sulfuric acid and hydrochloric acid).
1.6 Characterization of solvents
A special place in the characterization of solvents is occupied by the dielectric constant. The advantage of the latter over other criteria is related to the simplicity of electrostatic models of solvation, and therefore the dielectric constant has become a useful measure of solvent polarity. In this regard, it is important to clearly know what exactly reflects the macroscopic permeability of the solvent, also called the relative permittivity:
r = 0 /
0 is the dielectric constant of vacuum.
The dielectric constant is determined by placing a solvent between two charged capacitor plates. In the presence of a solvent, the electric field strength between the plates E decreases compared to a voltage E 0 measured in vacuum, and the ratio E 0 / represents the dielectric constant of the solvent. If the solvent molecules do not have their own constant dipole moment, then under the influence of an external field the intramolecular charges separate, inducing a dipole.
In an electric field, molecules with a constant or induced dipole are oriented in a certain way; this phenomenon is called polarization. The higher the degree of polarization, the stronger the decrease in electric field strength. Therefore, the dielectric constant is directly related to the ability of the solvent to separate charges and to orient its own dipoles.
1.6.1 Classification of solvents by physical properties.
The dielectric constant (DP) of solution e is one of the most important factors among those that have the strongest influence on the characteristics of a process occurring in a solution.
The DP values \u200b\u200bof individual solvents vary in a very significant range - from? 1.8 (alkanes) to 170-180 (N-alkylamides of aliphatic carboxylic acids). The terminology that underlies the gradation of solvents according to DP is not defined and consistent in everything. High DP solvents are called polar. and even high polar, although, strictly speaking, the term “polar” refers to the dipole moment µ of the solvent molecule, that is, it characterizes not the macro-but micro-properties of the solvent.
However, as follows from the theory linking with µ, in the general case there is a symbatic change in these quantities. In addition, to characterize solvents by the presence or absence of a dipole moment in their molecules, the terminology “dipole" (µ0) and “apolar” (µ \u003d 0) solvents.
Solvents whose DP is in the range \u003d l, 8h12 are classified as low-polar, solvents with \u003d 12h50 are medium-polar, and \u003e 50 are highly polar; this division is related not only and not so much to these rather randomly selected intervals, but also in a much more significant circumstance.
Despite the connection between the DP and the dipole moment of an individual liquid, which is quite clearly established by the theory, caution should be exercised against categorically unambiguous parallelism between the quantities and μ . The fact is that antiparallel (but not chain) association of dipoles leads to a decrease in the dipole moment of associates in comparison with the dipole moment of a single molecule and, consequently, to a drop in the liquid DP. That is why the DP of liquids formed by molecules with a relatively large dipole moment can be quite low.
A significant influence on the polarity of the solvent is exerted by its structural features. Structured solvents, as a rule, have a high DP.
The dielectric constant, along with the formation of homomolecular chemical bonds, is one of the most important factors determining the properties of individual liquids, which depend on the energy of intermolecular interaction. The latter is determined by the reactive interaction, the energy of which depends on the static DP (), the DP extrapolated to an infinitely long wavelength ( ) and taken equal to? 1,1 n D, as well as the dipole moment µ and molar volume ?:
E R \u003d 
The values \u200b\u200bof the reactive energies of the dipole molecules of a number of solvents are given in Table 1.
As emphasized by M.I. Shakhparonov, the reactive field of polar molecules does not lead to the formation of chemical structures (homomolecular associates), the existence of which is determined by chemical interactions. The stability of the latter decreases with increasing reactive field energy.
The reactive field energy explains a number of physical features of solvents, and therefore, it can be useful to classify solvents by value - Er .
Obviously, all solvents whose molecules do not have a dipole moment will constitute a class of non-reactive liquids. Solvents with reactive energy in the range - Er = 0h5 kJ · mol -1 constitute a low reactive class, in the range Er \u003d 5ch15 kJ mol -1 medium-reactive and, finally, solvents with - E R 25 kJ mol -1 constitute a class highly reactive solvents.
As a rule, highly reactive liquids dissolve both non-electrolytes and electrolytes well, and the latter dissociate better in them compared to other classes (according to this classification system) of solvents. This is explained by the fact that highly reactive solvents combine high values \u200b\u200bof DP and dipole moment.
table 2
The reactive energy of some solvents at 298.15 K.
| Solvent | -E R, kJ mol -1 | solvent | -E R, kJ mol -1 |
| Aniline | 2,9 | Methyl ethyl ketone | 3,4 |
| Acetone | 9,1 | Formic acid | 5,2 |
| Acetonitrile | 20,9 | Nitrobenzene | 17,1 |
| Butyl alcohol | 2,9 | Nitromethane | 17,5 |
| Water | 18,6 | Pyridine | 5,7 |
| Hexene 1 | 0,15 | Propylene carbonate | 43,8 |
| N, N-dimethylacetamide | 15,8 | Propyl alcohol | 3,6 |
| Dimethyl sulfoxide | 6,4 | Toluene | 0,05 |
| Dimethylformamide | 19,5 | Acetic acid | 3,7 |
| Dioxane | 0,07 | Tetrahydrofuran | 2,9 |
| 1,2 - dichloroethane | 4,2 | Chlorobenzene | 1,8 |
| about - Xylene | 0,12 | Ethyl acetate | 2,45 |
| n - Xylene | 0,05 | Ethylene glycol | 8,9 |
| N-methylacetamide | 24,1 | Ethanol | 4,7 |
| Methyl formate | 4,0 |
By viscosity (more precisely, by viscosity coefficient ) liquids are divided into low viscosity with яз 10 -2 Pa s.
Conductivity liquids are divided into “conductive” liquids with about 10 2 - 10 -1 cm · m -1 and above; “Moderately conductive” - with about 10 -1 - 10 -4 and with 10 -4 cm · m -1 is referred to as “non-conductive”.
Mention is also made of the classification of solvents by boiling point, in which solvents are divided into low boilers (1 50 0 С).
For the classification of solvents by their ability to evaporate, a relative scale based on the heats of evaporation is proposed. Therefore, volatile (relative volatility 35) solvents are distinguished. If we focus directly on the heats of evaporation, then to the volatile should be attributed solvents characterized by the heat of vaporization Table 3
Classification of solvents by dielectric constant
| STRONGLY POLAR |
|||
| SOLVENT |  r r | SOLVENT | r |
| Water | 78,30 | DMF | 36,71 |
| Ethylene glycol | 37,7 | Benzonitrile | 26,20 |
| Methanol | 32,66 | Nitrobenzene | 34,78 |
| DMSO | 46,45 | Glycerol | 51,7 |
| WEAKLY POLAR |
|||
| THF | 7,58 | Acetic Anhydride | 20,7 |
| Diethyl ether | 4,20 | Butanon - 2 | 18,51 |
| Acetone | 20,70 | Pentanone - 2 | 15,38 |
| Ethanol | 24,55 | Pyridine | 12,91 |
| Acetic acid | 6,17 | Methyl acetate | 6,68 |
| Propanol - 1 | 20,45 | Cyclohexanone | 16,10 |
| Butanol - 1 | 17,51 | Quinaline | 8,95 |
| Isoamyl alcohol | 15,19 | Chloroform | 4,81 |
| Aniline | 6,71 | Amyl alcohol | 13,9 |
| Nonpolar |
|||
| 1,4 - dioxane | 2,21 | Cyclohexane | 2,02 |
| Trichlorethylene | 3,42 | N - pentane | 1,84 |
| Benzene | 2,27 | Carbon disulfide | 2,64 |
| Toluene | 2,38 | Diethylamine | 3,78 |
| Tetrachloromethane | 2,23 | diethyl carbonate | 2,82 |
| Triethylamine | 2,42 | ||
| n - heptane | 1,92 | ||
| n - hexane | 1,88 | ||
1.6.2 Solvent classification systems based on their chemical properties
Most systems for classifying solvents by chemical characteristics in explicit or implicit forms take into account their acid-base properties. Therefore, the most common solvent classification system involves dividing them into two broad classes - donor
(basic) and acceptor (acid), the definition of which, as is obvious, predetermines the allocation of another class of indifferent solvents. The conventionality of classifying each particular solvent as one of these classes, if only because the ability of the solvent molecule to give or receive an electron pair from a partner, depends on the properties of both the solvent and the dissolved compound. That is why this classification provides for the characteristic behavior of a solvent in reactions or solvation processes.
A common feature of donor solvents is the predominant solvation of cations as particles characterized by electron deficiency. Similarly, acceptor solvents predominantly solvate anions as particles with an excess of electrons.
The variety of donor solvents is determined by the relatively large number of elements, the atoms of which can act as donors of the electron pair, and the variety of chemical compounds of these elements. Representatives of this class of solvents are therefore subdivided into N-bases (amines or amides of various types), 0-bases (ethers and esters, alcohols, ketones, less often aldehydes), S-bases (thioethers, thioalcohols, sulfoxides), P-bases (trialkyl, triaryl or alkylarylphosphines) and the like.
Acceptor solvents are divided into protic and aprotic.
Solvents capable of participating in protolytic equilibrium, depending on their function, are often called protophilic (basic) and protogenic (acidic). Solvents equally willingly exhibiting both functions (alcohols, ketones) are called amphiprotic.
The molecules of many solvents are involved in solvation through the formation of a hydrogen (H-) bond. In accordance with this, a classification of solvents by their ability to form an H-bond is proposed.
The first class in this classification system is composed of liquids in which a three-dimensional network of H-bonds exists. Solvents of this class (water, formic acid, sulfuric acid, glycols) are characterized by a very high DP and relatively high viscosity and activation energy of a viscous flow. As a rule, solvents of this class dissolve well in each other, forming heteromolecular associates through the H-bond.
The second class is liquids with a two-dimensional network of H-bonds. As a rule, solvent molecules of this class contain one hydroxyl group (monohydric alcohols, monobasic carboxylic acids, phonols, etc.). The desire to form heteromolecular associates in this group of solvents is generally less pronounced than in representatives of the first class.
The third class unites liquids whose molecules include nitrogen, oxygen, sulfur, fluorine, etc. atoms capable of forming an H bond with proton donors. Ethers, amines, ketones, aldehydes, etc. are included in this class.
The fourth class includes liquids whose molecules can be proton donors. These include chloroform, dichloroethane, etc.
The fifth class unites liquids whose molecules are not capable, under ordinary conditions, of participating in the formation of an H-bond. This group includes alkanes, carbon tetrachloride, perhaloalkanes, etc.
Parker proposed classifying solvents by their ability to solvate ions. Within this classification, solvents are divided into groups.
Apolar aprotic - liquids with low DP (
Dipolar aprotic - liquids with relatively high DP values \u200b\u200b(\u003e 15) and dipole moments (μ\u003e 2D) ,
not containing hydrogen capable of forming an H-bond. Representatives of this group of solvents are sulfur dioxide, nitrobenzene, nitromethane, acetonitrile, propylene carbonate, DMSO, etc.
Protonic - liquids whose molecules contain groups where the hydrogen atom is connected to an electronegative atom. Compounds included in this group (carboxylic acids, alcohols, phenols, etc.) are distinguished by a pronounced ability to form an H-bond.
Solvating ability of solvents generally increases from the first group to the third.
Bronsted proposed classifying solvents based on a combination of permittivity and protogenicity. In accordance with these characteristics, he divided the solvents into eight groups.
Examples of solvents of various groups within the framework of the Bre'nsted classification are: I — water; II — sulfuric acid, formic acid; III — hydrazine, DMSO; IV — propylene carbonate, nitrobenzene, acetonitrile, nitromethane; V — butanol; VI — liquid bromine and hydrogen iodide; VII - triethylamine, pyridine; VIII - hexane, chlorobenzene.
The Bronsted classification allows us to predict the acid-base properties of dissolved compounds in the solvents of each group. So, in a solvent of group II, the strength of the bases is significantly higher than in a solvent of group III; the strength of acids in group III solvents is generally higher than in group VII solvents, etc.
Table 4
Bronsted solvent classification.
| Property | Solvent type |
|||||||
| I | II | III | IV | V | VI | VII | VIII |
|
| Dp | + | + | + | + | - | - | - | - |
| Protogenicity | + | + | - | - | + | + | - | - |
| Profuse | + | - | + | - | + | - | + | - |
* The signs + and - in the first line correspond to high and low DP, in the remaining lines to the presence or absence of this property.
A classification of solvents is also proposed based on eight physical characteristics: the Kirkwood function, molar refraction, Hildebrand's solubility parameter, refractive index, boiling point, dipole moment, as well as the energies of higher filled and lower unfilled molecular orbitals. By the combination of these properties, it is proposed to subdivide the solvents into nine groups:
1) aprotic dipolar (e.g. acetonitrile, nitromethane, acetone, 1,2-dichloroethane);
2) aprotic highly dipolar (DMSO, DMF, DMAA, propylene carbonate, nitrobenzene);
3) aprotic, highly dipolar and highly polarizable (sulfolane, HMFTA);
4) aromatic non-polar (benzene, toluene);
relatively polar aromatic (chlorobenzene, o-dichlorobenzene, acetophenone);
electron-donating (triethylamine, ethers, dioxane);
H-bond solvents (alcohols, carboxylic acids);
strongly associated solvents with an H-bond (formamide,
diethylene glycol, water);
solvents with uncertain function (carbon disulfide, chloroform).
1.7 Overview of solubility solids
1.7.1 Gravimetric solubility determination
The method consists in determining the mass of a substance dissolved in a certain mass of solvent (specific solubility P ` ) and the calculation of solubility as a mass of a substance capable of dissolving in 100 grams of a given solvent. The method is an evaluation.On a technochemical balance, a sample of the test substance is weighed (g 1 ` ) Weigh the solvent (g p). The dry filter mass (g 2) is determined. Pour a sample of the substance into the solvent, if the substance is completely dissolved, it is necessary to add it until the dissolution stops, note the full sample of the substance poured into the solvent (g 1). Filter out the insoluble matter through the filter and determine the mass of the wet filter with residual substance (g 3). Dry the filter with the remainder of the substance to constant weight (g 4). Calculate the specific solubility by the formula:
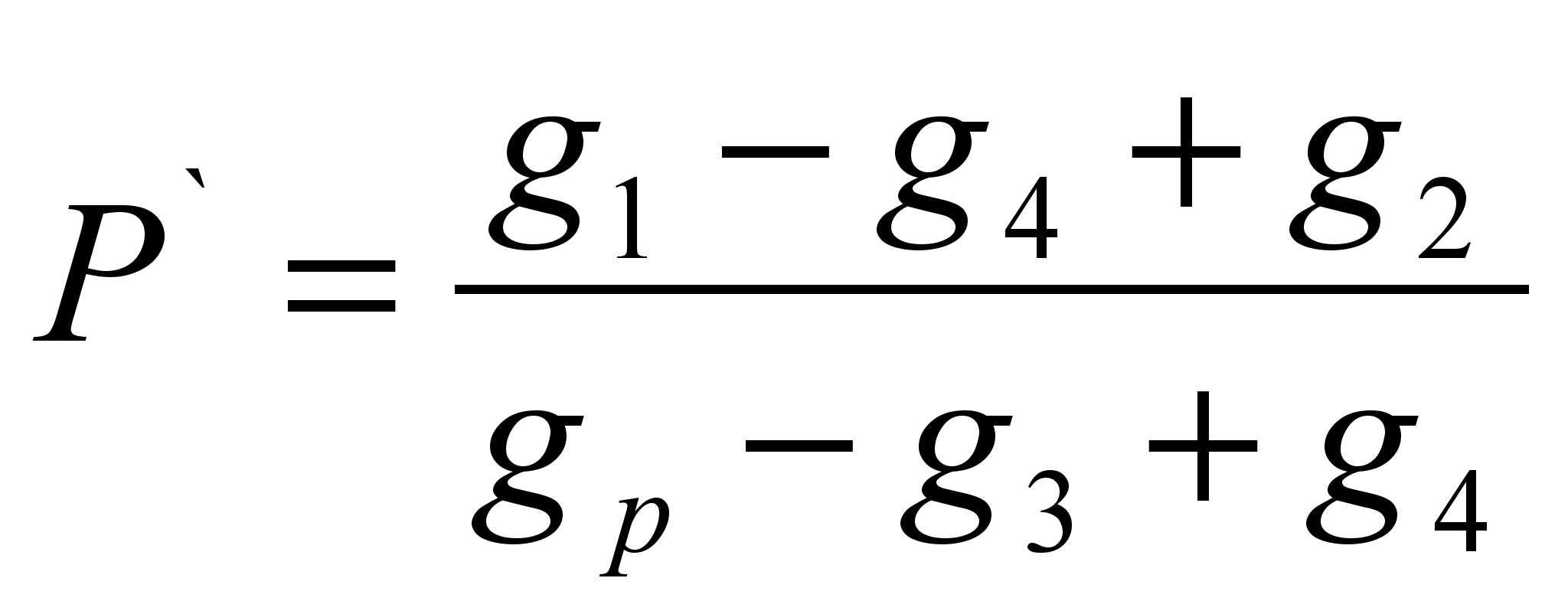
P \u003d P`100 (g / 100 g)
Table 5
Solubility of phenolphthalein in some solvents
| solvent | solubility | r |
| Highly polar |
||
| Water | 0,0002 | 78,30 |
| Methyl alcohol | 19,42 | 52,66 |
| Weakly polar |
||
| Ethanol | 17,29 | 24,55 |
| Acetone | 16,98 | 20,70 |
| chloroform | 2,97 | 4,81 |
| Not polar |
||
| xylene | 0,19 | 2,43 |
| toluene | 0,17 | 2,38 |
| benzene | 0,16 | 2,27 |
1.7.2 Electrochemical method.
1. Assemble the installation to determine the resistance of the volume of the electrolyte solution according to the scheme (see Fig. 3).
2. An aqueous solution of potassium chloride of a certain concentration is poured into the measuring vessel to the mark and the vessel with the electrodes is kept in the thermostat at a given temperature. After 15-20 minutes, connect the vessel at points B and D to the installation.
3. Using the resistance store and moving contact C, the bridge is balanced, achieving either the smallest sound in the phone or the minimum amplitude of the sine wave on the oscilloscope screen. The compensation point is approached either from one end of the rechord, or from the other. Measurement and subsequent calculation of R x:
R x \u003d R 1 
Repeat 3-4 times at different resistances R 1. In this case, the movable contact should not approach the ends of the reochord. If the deviations in the measurements are less than 0.5%, then the results are considered satisfactory and proceed to the calculation of the constant vessel by the formula:
K \u003d  /
w =
R x
/
w =
R x
Where R x is the resistance of the solution; -
electrical conductivity.
4. A small amount of salt is poured into 100 ml of water and, tightly closing the flask with a stopper, continuously shake it for 20 minutes. The resulting suspension is sucked off through a glass filter, and the precipitate is used to prepare a saturated solution. The resulting solution is poured into a vessel for measuring electrical conductivity and after thermostating, the resistance of the solution is measured.
=
k /
R x  = /
C
= /
C
Where C is the normality of the solution, kg eq / m 3.
Since the salt concentration in the saturated solution is low, then \u003d . Based on the equation:
C \u003d ( r - in ) /
Where r - the electrical conductivity of the salt solution.
Solubility product for monovalent electrolyte:
![]() = (C ) 2
= (C ) 2
6. The results are listed in the table:
| salt | t, C 0 | cm / m | Resistance, Ohm | K | | | C | L |
|||
| R 1 | R 2 (AC) | R 3 (CB) | R x |
||||||||
Table 6
KCI solution conductivity
| t, C 0 | 1 N KCI | 0.1 N | 0.02 N | 0.01 N |
| 10 4, Ohm -1 cm -1 |
||||
| 0 | 654,1 | 71,5 | 15,21 | 7,76 |
| 10 | 831,9 | 93,3 | 19,94 | 10,20 |
| 14 | 906,3 | 102,5 | 21,93 | 11,21 |
| 16 | 944,1 | 107,2 | 22,94 | 11,73 |
| 18 | 982,2 | 111,9 | 23,97 | 12,25 |
| 20 | 1020,7 | 116,7 | 25,01 | 12,78 |
| 22 | 1055,4 | 121,5 | 26,06 | 13,32 |
| 24 | 1098,4 | 126,4 | 27,12 | 13,86 |
| 25 | 1118,0 | 128,8 | 27,65 | 14,13 |
| 26 | 1137,7 | 131,8 | 28,19 | 14,41 |
| 28 | 136,2 | 29,27 | 14,96 |
|
| 30 | 141,2 | 30,36 | 15,52 |
|
| 32 | 146,2 | 31,46 | 16,09 |
|
| 34 | 151,3 | 32,56 | 16,67 |
|
| 36 | 156,4 | 33,68 | ||
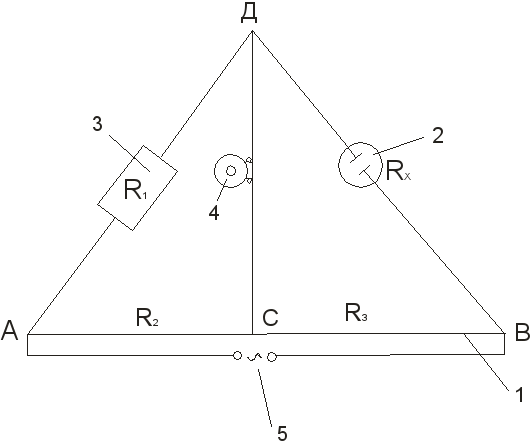
Fig.3 Installation diagram for determining electrical conductivity:
1 - wire stretched on a ruler; 2 - measuring vessel with electrodes; 3 - rheostat; 4 - low resistance telephone; 5 - current source.
1.7.3
Photoelectrocalometric method.
The method is based on the law of optical density additivity subject to the basic law of light absorption.
Working process
1. The choice of optimal filters.
1.1. Place in a 25 ml volumetric flask:
Stir the solutions and measure the optical densities with respect to the selected solvent at various filters in the most suitable cuvettes for this. The size of the cuvette is indicated. The method of work on the FEC, see the instrument passport.
1.2. Record the obtained data in the table:
1.3. On graph paper, construct light absorption curves A \u003d f (, nm) and determine which filters with which wavelengths are best used when working.
2. Charting.
2.1. Prepare a series of standard solutions. Measure the optical density of each prepared series of standard solutions with previously selected filters.
2.2. Enter the experimental data in the table (see below) for each series of solutions separately, carry out statistical processing of the results.
2.3. Using tabular data on graph paper, plot A \u003d f (C of the substance) graphs and calculate the solubility of phenolphthalein in solvents.
Having studied the literature on the solubility of phenolphthalein, the methods mentioned above were selected. And comparing them for the possibility of using them in our laboratory conditions, for the duration of the test, for the simplicity of their implementation, the gravimetric solubility determination technique was chosen.
2. The experimental part
2.1 Synthesis of phenolphthalein
The synthesis was carried out according to the method specified in the literature review (paragraph 1.4.1).
| Act | observation |
| 1. Replied: 5 g phthalic anhydride | White crystalline powder Pinkish, blurry crystals |
| 2. I placed the reagents in a flat-bottomed conical flask (per 100 ml), stirred it with a thermometer. | A mushy mass formed, a slight heating of the mixture occurs (t? 37 0 C). |
| 3. Put the flask heated in a sand bath (temperature within 125 - 130 0 С). Heated for 1.5 hours. | At a temperature of 45 ° C, phenol melts, phthalic anhydride (white flakes) floats in a brownish melt. After heating for 30 minutes (t? 130 0 C), a uniform brown - raspberry melt was formed. After 1 hour of heating - a homogeneous melt of blood-red color. After 1.5 hours, the heating stopped. |
| 4. Poured hot melt into a flask with boiling water and distilled unreacted phenol with water vapor (for the installation drawing, see the corresponding section of the literature review). | After pouring hot melt in a flask with boiling water, light yellow pieces of phenolphthalein began to float, a slight turbidity of the water occurs, t? 99 ° C. A cloudy distillation takes place (V \u003d 50 ml). After 15 minutes of distillation, a clear distillate began to drip. |
| 5. Decanted the solution with phenolphthalein to the Buchner funnel without transferring the precipitate to the filter. Washed the precipitate with three portions of cold water, 100 ml each. | The temperature of the solution is 26 0 С, in the solution (V? 170 ml) grains-like pieces of phenolphthalein are slightly yellowish. |
| 6. Dissolve filtered phenolphthalein in 15 ml of a warm solution of sodium hydroxide (? \u003d 5%). | T NaOH? 42 0 С, staining of the solution occurred - a dark red color. |
| 7. Conducted a filtration of a dark red solution. | The temperature of the solution? 35 0 С. After the end of the filtering, the donkey has a thin, pink-white, finely crystalline layer of matter (most likely this is not reacted phthalic anhydride). |
| 8. To the filtrate (V? 50 ml) added 5 ml of acetic acid (c.), In 1 ml portions, stirring. | The color of the solution changed from dark red to gray-white, cloudy (flocculent) solution. The heating of the solution did not occur. The temperature of the solution? 31 0 C. |
| 9. Added 2 drops of hydrochloric acid (K.) | No visual changes |
| 10. Left to stand for 1 hour. | The flakes settled on the bottom of the glass, a clear solution was above the precipitate, the flakes were grayish-white. |
| 11. Filtered the resulting precipitate. | The filter is a white-grayish slurry. M ow? 5.07 g. |
A total of 5.07 g of wet preparation was obtained.
2.2 Recrystallization from hot alcohol
Recrystallization was carried out according to the procedure indicated in the literature review (paragraph 1.4.4). One repetition was performed. The device drawing is shown in paragraph 1.4.4 of the literature review.
| Act | Observation |
| A portion of phenolphthalein (5.07 g) was placed in a flask and added 50 ml of 96% ethanol. | A small portion of pheolphthalein has dissolved. Alcohol became slightly cloudy. |
| He heated the flask to a slight boil. Added alcohol until the phenolphthalein is completely dissolved (0.5 ml each). V ETOH \u003d 7 ml. | phenolphthalein is completely dissolved. Alcohol is unclear. t mixture \u003d 85 about C. |
| The mixture was slowly cooled to room temperature. | t mixture \u003d 19 about C. Many white crystals fell out of solution in the form of small crystals. The solution is clear. |
| The precipitated crystals were sucked off and, washing on the filter with ice water, dried between sheets of filter paper. | phenolphthalein obtained in the form of white-gray air crystals, fine-grained. m (phenolphthalein) \u003d 4.08 g. The recrystallization yield was 83.5%. |
Recrystallization calculation:
Recrystallization Output:
W,% \u003d (m (phenolphthalein) cross-dry 100%) / m (phenolphthalein) ow
W,% \u003d  % = 83,5 %
% = 83,5 %
Synthesis yield calculation:
To obtain 0.0388 mol of phenolphthalein (theoretical) or m \u003d 0.0338 mol 318 g / mol \u003d 10.7 g.
The output from the theoretical:
W \u003d  100 % = 40, 5 %
100 % = 40, 5 %
Thus, in principle, a sufficient amount of phenolphthalein (about 4.08 g) was obtained for further research. The synthesis was carried out with an acceptable yield.
2.3 Determination of solubility
The solubility of phenolphthalein was determined by gravimetry (see paragraph 1.7.1).
Chloroform, benzene and xylene were selected as solvents. since according to the literature (see table 5), phenolphthalein in them has acceptable solubility for the available mass of phenolphthalein. I did not use water, since phenolphthalein has a very small solubility (about 10 -4 g per 100 g). I also did not use ethanol, because phenolphthalein has a very good solubility in alcohol and a large consumption of the substance will be required. The determination was carried out at 21 ± 1 о С (temperature of the liquid phase). Infusion lasted 30 ± 1 min, with vigorous stirring.
Calculation Formulas:
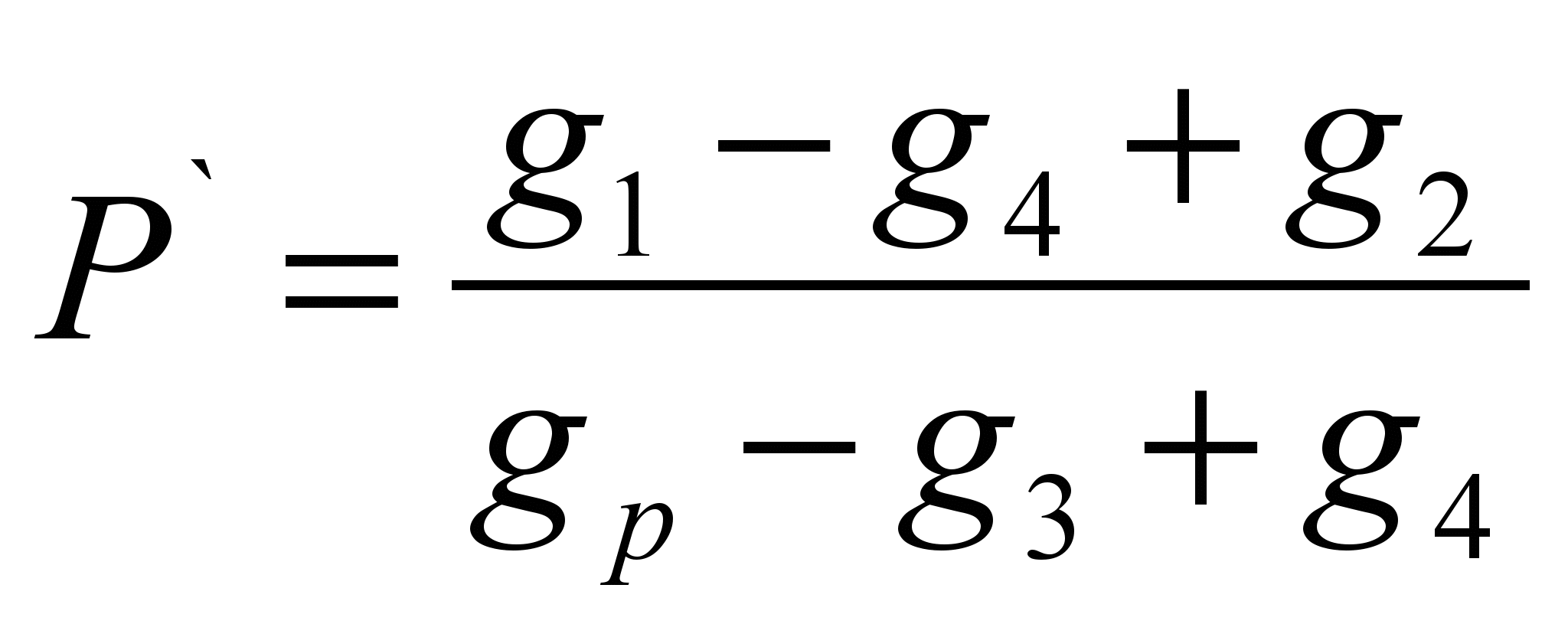 ; P \u003d P`100 (g / 100 g)
; P \u003d P`100 (g / 100 g)
Where, g 1 - a portion of phenolphthalein, g;
G 2 - dry filter mass, g;
G 3 - the mass of the wet filter with phenolphthalein residues, g;
G 4 - dry filter mass with phenolphthalein residues, g;
G p - sample of the solvent, g;
P - solubility, g / 100 g sol;
P` - specific solubility, g / 1 g sol.
Definition Results:
P (phenolphthalein / chloroform) \u003d (3.0 ± 0.03) g / 100 g of chloroform; ? \u003d 1%.
P (phenolphthalein / xylene) \u003d (0.22 ± 0.01) g / 100 g xylene; ? \u003d 6%.
P (phenolphthalein / benzene) \u003d (0.19 0.01) g / 100 g benzene; \u003d 6%.
According to the experiment, a pivot table and a graph are built.
Table 7
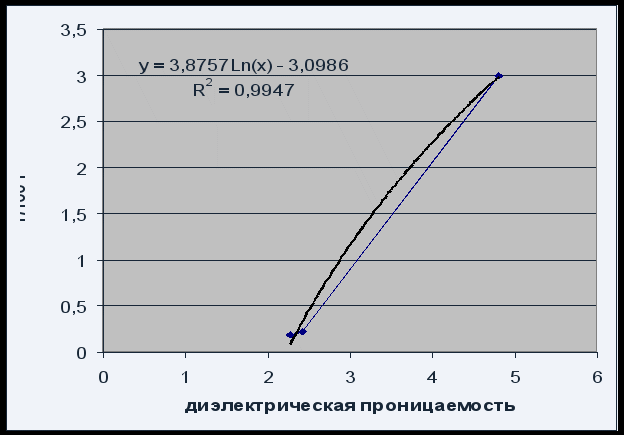
Fig. 4 Solubility versus solvent dielectric constant
The dependence of solubility on the dielectric constant of the solvent can be described by the logarithmic equation P \u003d 3.8757 Ln (x) - 3.0986 with a satisfactory quality of R 2 \u003d 0.995.
Due to the fact that the solubility of phenolphthalein increases with increasing dielectric constant of the solvent (increasing its polarity), we can say that phenolphthalein is a polar compound.
Conclusion
In the course of the work, 26 literary sources were analyzed. The general position of the preparation of phenol and phthalic anhydride using the reaction is described. A review of the synthesis methods was carried out (one method was found, in different literature sources the method differed in the mass of reagent loading) of phenolphthalein. The synthesis according to this technique is not complicated by side processes. The main hazards during the synthesis and handling of the substances used are determined. Methods for determining solubility were selected. Chloroform, xylene and benzene were chosen as solvents for determining solubility.
The synthesis of phenolphthalein was carried out with a yield of 40.5%. The conditions of synthesis, purification and isolation were observed in strict accordance with the data of the methodology.
The solubility of phenolphthalein (according to gravimetric determination) was: 3.00 ± 0.03 g / 100 g of chloroform, 0.22 ± 0.01 g / 100 g of xylene, 0.19 ± 0.01 g / 100 g of benzene.
An increase in the solubility of phenolphthalein with an increase in the dielectric constant of the solvent confirms that phenolphthalein is a polar compound.
List of references
1.Agronomov A.E. laboratory work in organic chemistry. - M .: Education, 1977.230 s.2.Astakhova L.I., Krivenko A.P. Workshop on Organic Chemistry. - M .: Mir, 1990.180 s.
3. Bishot E. Indicators. - M.: Mir, 1976.356 s.
4. Buler K., Pearson D. Organic syntheses. M .: Higher. Shk., 1973.340 s.
5.Gerasimov Y.I. Thermodynamics of solutions. - M.: Publishing House of Moscow State University, 1980.170 s.
6.Gitis S.S. Workshop on Organic Chemistry / S. S. Gitis, A.I. Glaz, A.I. Ivanov. - M .: VSH, 1991.
7.Golodnikov G.V. Workshop on Organic Chemistry. - M.: Chemistry, 1978.160s.
8.Grandberg I.I. Practical work and seminars in organic chemistry: A manual for students. universities. - 4th ed., Revised. and add. - M.: Bustard, 2001.
9. Ivanov V.G. Workshop on Organic Chemistry: Textbook. Manual for students. higher ped textbook. institutions / B. G. Ivanov, O. N. Geva, Yu. G. Gaverova. - M.: Publishing Center "Academy", 2000.
10.Karapetyants M.Kh. Introduction to the theory of chemical processes. - M .: Higher. Shk., 1975.280 s.
11.Kirilin V.A. Thermodynamics of solutions. - M .: Energy, 1980.80 s.
12.Kogan V.B. Solubility Guide. - M.: L. Publishing House Academy of Sciences, 1961.300 s.
13. Levina R. Ya. Practical work in organic chemistry. - M.: Chemistry, 1980.
14.Organicum: Practical work in organic chemistry. - M .: Mir, 1979 - V.2.
15.Petrov A.A. Organic chemistry: Textbook for high schools / A. A. Petrov, H.V. Balyan, A.T. Troshchenko. - 5th ed., Revised. and add. - St. Petersburg: Ivan Fedorov, 2002.
16. Raykhard K. Solvents and environmental effects in organic chemistry. - M.: Mir, 1991.182 s.
17. A guide to laboratory classes in organic chemistry: A manual for universities / N. N. Artemyev, V. L. Beloborodova, S. E. Zurabyan and others; Ed. N. A. Tyukavkina. - 2nd ed., Revised. and add. - M.: Bustard, 2002.
18. Properties of organic compounds: reference book / Ed. A.A. Potekhin. - L .: Chemistry, 1984.
19. Smolina T. A. Practical work in organic chemistry: a small workshop / T. A. Smolina, N.V. Vasilieva, N. B. Kupletskaya. - 2nd ed., Revised. - M .: Education, 1986.
20. Handbook of a chemist / Ed. B.N. Nikolsky and others .-- L .: Chemistry, 1964.
21.Stromberg A.G. Physical chemistry: Textbook. for chem. specialist. universities / A. G. Stromberg, D.P. Semchenko; Ed. A. G. Stromberg. - 5th ed., Rev. - M .: VSH, 2003.
22.Traven V.F. Organic chemistry: Textbook for high schools. - M.: IKC "Akademkniga", 2004. - T.1.
23. Physical chemistry. In 2 book Prince 1. The structure of the substance. Thermodynamics: Textbook. for universities / K. S. Krasnov, N.K. Vorobyov, I.I. Godnev and others; Ed. K. S. Krasnova - 3rd ed., Rev. - M .: VSH, 2001.
24.Fialkov Yu.A. Solvent as a means of controlling a chemical process. - L .: Chemistry, 1990.240 s.
25. Chemical Encyclopedic Dictionary / Ed. I. L. Knunyantsa et al. - M .: Soviet Encyclopedia, 1983.
26. Khramkina M.N. Workshop on Organic Synthesis. - M.: Publishing House of Moscow State University, 1985.473 s.
The empirical formula of Phenolphthalein is: C20H14O4 .
What is phenolphthalein?
According to Wikipedia, 4,4′-dioxophthalophenone or 3,3-bis- (4-hydroxyphenyl) phthalide represents acid base indicator .
Substances such as litmus, phenolphthalein, methyl orange widely used in chemistry to determine the acidity of solutions.
In an unchanged form, the agent is a transparent crystal that is poorly soluble in water, but soluble in alcohols and diethyl ether . You can synthesize a substance from phenol and phthalic anhydride using a condensation reaction using zinc chloride as catalyst (you can still use concentrated sulfuric acid ).
This substance in an acidic environment ( pH from 0 to 3) acquires a pronounced orange hue. In slightly acidic and neutral environments ( pH from 4 to 7) the solution will not change its color. Using Phenolphthalein, an alkaline medium can be recognized. Since the product acquires a raspberry color in solution, pH which from 8 to 10 (alkaline solution). If the values hydrogen indicator from 11 to 14, then the indicator will not affect the color of the drug. Phenolphthalein is used for titration various aqueous solutions in analytical chemistry usually a substance dissolved in alcohol is used.
Phenolphthalein has also been widely used in medicine. What Purgen ? It is a synonym for phenolphthalein. In the last century, the substance was actively used as a laxative. The drug actively stimulates intestinal motility. Now Purgen-laxative is rarely used, due to its ability to accumulate in the body and adversely affect the functioning of the kidneys.
pharmachologic effect
Laxative.
Pharmacodynamics and pharmacokinetics
Phenolphthalein, what is it?
Purgen is a powerful laxative. The mechanism of its action is based on strengthening peristalsis intestines. This is due to the ability of the substance to inhibit sodium potassium ATPase by stimulating adenylcyclase and raising biosynthesis . The substance stimulates the synapses and nerve endings of the intestinal walls, is disturbed water-electrolyte balance in Gastrointestinal tract fluid accumulates.
After the first dose, the effect of the drug occurs within a day. The substance has the property of cumulating in the body, has an irritating effect on the kidneys, has carcinogenic properties.
Indications for use
Preparations containing this substance are used as a laxative for chronic.
Contraindications
Laxative Purgen is contraindicated in:
- with kidney disease;
- patients with intestinal obstruction ;
- when on phenolphthalein;
- if a patient has a complex of symptoms “ sharp belly ”.
The medicine can not be used for a long time. Particular care must be taken when treating with the elderly.
Side effects
Purgen tablets can cause:
- heart palpitations due to disturbance water-electrolyte balance ;
- albuminuria ;
- collapse ;
- decreased potassium in the blood;
- discoloration of urine from yellow to pink or brown;
- skin rashes and.
Instructions for Purgen (Method and dosage)
Laxative is released in the form of tablets, various dosages or powder for oral administration.
Instructions for use Phenolphthalein
The average daily dosage for an adult is 100 mg.
Children are prescribed 50-200 mg of the drug per day, depending on their age and weight.
The maximum amount of a substance that can be taken within 24 hours is 300 mg.
The course of treatment is prescribed by a specialist. Drugs containing this component can not be taken for a long time.
Overdose
Medicine can cause hemorrhoidal bleeding , a significant decrease, up to collapse the appearance of protein in the urine. In case of an overdose, it is recommended to consult a doctor.
Interaction
Phenolphthalein can lead to a delay in potassium in the body if therapy is carried out in parallel potassium-sparing diuretics .
Terms of sale
No recipe required.
Storage conditions
Phenolphthalein-based preparations are stored in a dry, dark place, protected from children, at room temperature.
Shelf life
The powder has an unlimited shelf life, tablets can be stored for 10 years (unless otherwise indicated on the packaging by the manufacturer).
special instructions
Now drugs containing phenolphthalein are rarely used in medicine. Most often, they prefer other modern, safe laxatives.
For children
The substance is prescribed with caution. A dosage adjustment is necessary, depending on the age and weight of the child.
Elderly
In elderly people, the effect of taking this drug occurs within 24 to 72 hours.
For weight loss
At the beginning of the last century, the substance was used to lose weight. Now there are other drugs that have a similar effect. Many girls in the weather for an ideal figure abuse laxatives, which should not be done. It can be dangerous to your health.
Preparations that contain (Analogs)
Matches for ATX code level 4:
Ex Lax, Purgen, Purgofen, Purgil, Phenaloin, Laxatol, Laxoyl.
Purgen price, where to buy
The price of laxative in the form of phenolphthalein powder in wholesale is about 1700 rubles per 1 kg.
Buying a drug in Moscow is almost impossible, since drugs based on it have not been available in pharmacies for a long time.
The price of phenolphthalein may vary, depending on the manufacturer.
